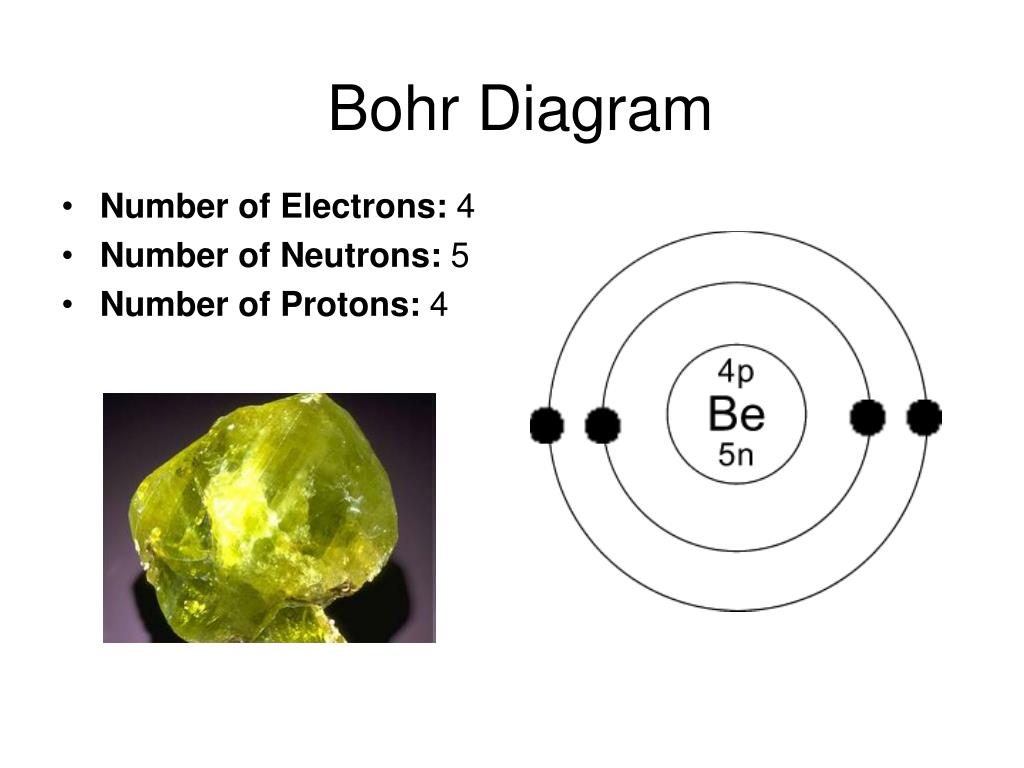
PPT Lithium, Beryllium, & Boron PowerPoint Presentation, free download ID5742588
Figure 6.19.1 6.19. 1 The figures above show the electron density of different elements. On the left from top to bottom are Hydrogen, Helium, and Lithium. On the right from top to bottom are Beryllium, Boron, and Carbon. Hydrogen has a larger circular area concentrated with dots when compared to helium.
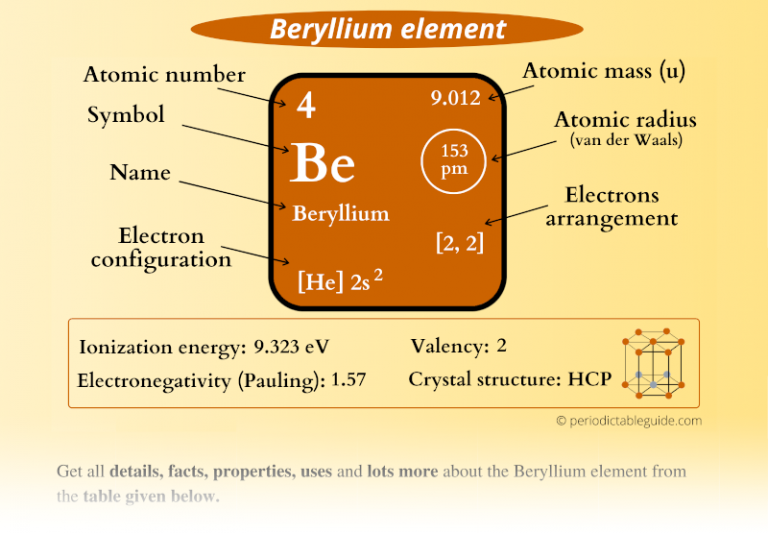
Beryllium Element in Periodic table (Info + Why in Group 2?)
Stars fuse hydrogen and helium into heavier nuclei. All stars produce carbon via the triple-alpha process. Carbon releases neutrons, which feed the slow neutron-capture or s-process.. 13.8 billion years ago: Hydrogen, helium, lithium, beryllium, and possibly boron formed in the first 20 minutes after the Big Bang. These are the primordial.

Name the elements from atomic no. 1 30 Brainly.in
Explanation: The elements Hydrogen and Helium were made during the Big Bang. Helium is also made by fusion reactions in stars. The elements Lithium Beryllium and Boron can't be made in any quantity is stars as they are intermediate steps in other fusion reactions.

An image of some of the basic elements from the periodic table hydrogen, helium, lithium
There′s Hydrogen and Helium Then Lithium, Beryllium Boron, Carbon everywhere Nitrogen all through the air With Oxygen so you can breathe And Fluorine for your pretty teeth Neon to light up the signs Sodium for salty times (Magnesium, Aluminium) Silicon (Phosphorus, then Sulfur) Chlorine and Argon (Potassium) And Calcium so you'll grow strong.
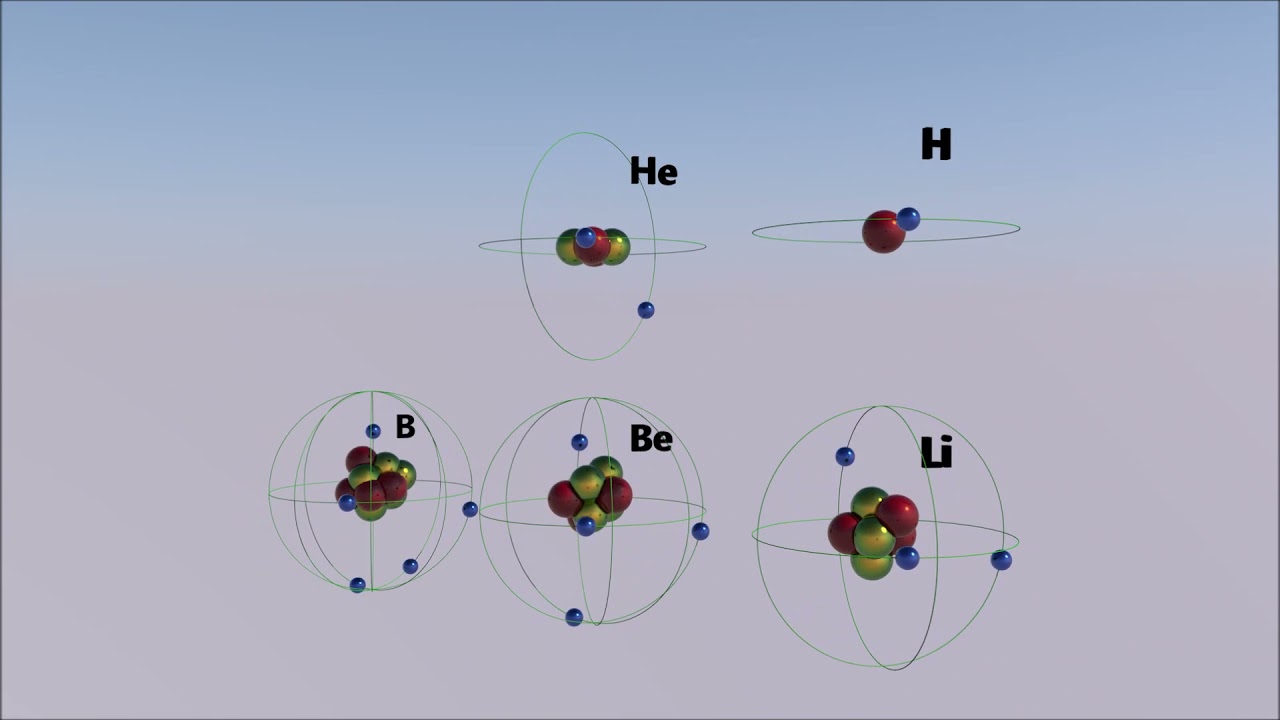
Atomic structure Hydrogen H, Helium He, Lithium Li, Beryllium Be, Boron B. YouTube
The first 30 elements of the periodic table: Hydrogen, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, Fluorine, Neon, Sodium, Magnesium, Aluminium, Silicon, Phosphorus, Sulphur, Chlorine, Argon, Potassium, Calcium, Scandium, Titanium, Vanadium, Chromium, Manganese, Iron, Cobalt, Nickel, Copper and Zinc. Explain It To A Child

Periodic Table Rows Labeled Periodic Table Timeline
1.. IntroductionLight element nucleosynthesis is an important chapter of nuclear astrophysics. Specifically, the rare and fragile light nuclei, lithium, beryllium and boron (LiBeB) are not generated in the normal course of stellar nucleosynthesis (except 7 Li, in the galactic disk) and are, in fact, destroyed in stellar interiors. This characteristic is reflected in the low abundance of these.

1S2 2S2 2P1 Boron Neon Lithium Helium Elements, Chemicals and Chemistry / In a quantum
The Elements of the Periodic Table.) There's Hydrogen and Helium. Then Lithium, Beryllium. Boron, Carbon everywhere. Nitrogen all through the air. With Oxygen so you can breathe. And Fluorine for your pretty teeth. Neon to light up the signs. Sodium for salty times.

Periodic Table First 20 Elements Chemistry Knowledge Easy English Learning Process YouTube
The 20 elements and their symbols are Hydrogen (H), Helium (He), Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne), Sodium (Na), Magnesium (Mg), Aluminum (Al), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (Cl), Argon (Ar), Potassium (K), and Calcium (Ca).

1 H Hydrogen 2 He Helium 3 Li Lithium 4 Be
First 20 Elements The first 20 elements of the periodic table have been tabulated below, along with their symbols and atomic numbers. Learn more ⇒ Interactive Periodic Table Table of Contents What Information does the Atomic Number of an Element Provide? Why is Potassium denoted by the symbol 'K' and Sodium by the symbol 'Na'? Recommended Videos

[Periodic Table] hydrogen helium lithium beryllium boron 30 elements YouTube
J. Linz Waveforms of the first 12 elements. From top to bottom, the left column shows hydrogen, helium, lithium, beryllium; the middle column shows boron, carbon, nitrogen, oxygen; and the right column shows fluorine, neon, sodium, magnesium. The sound of each atom can be heard here.
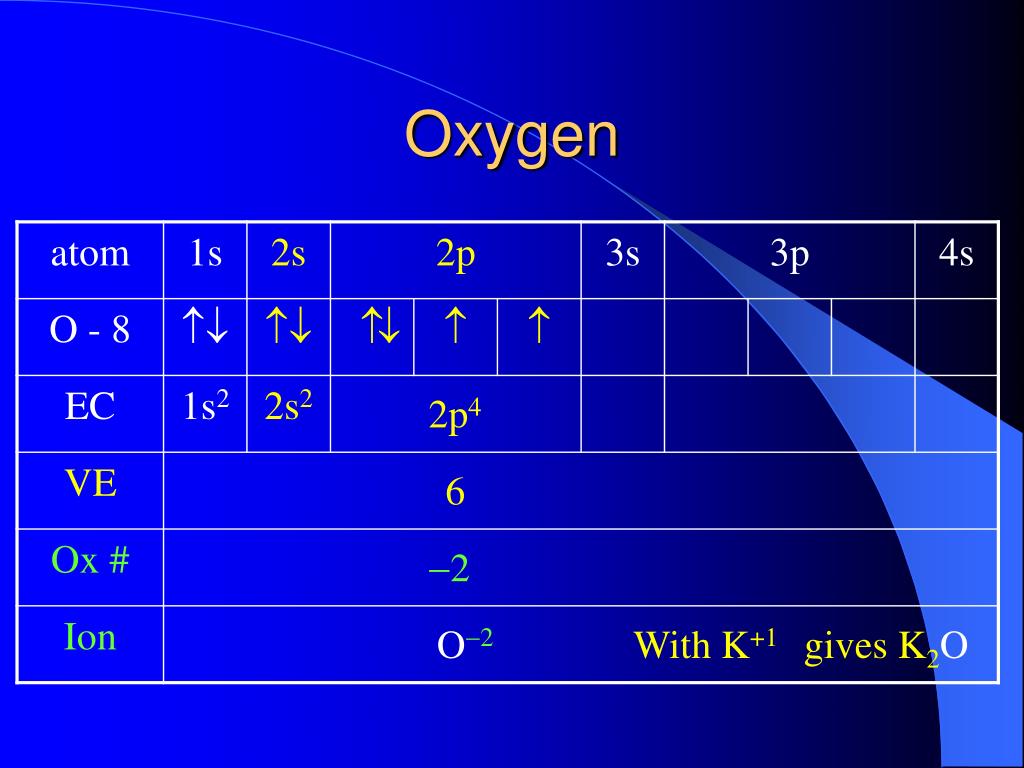
1S2 2S2 2P1 Boron Neon Lithium Helium Elements, Chemicals and Chemistry / In a quantum
Vol. 1, Section 1 : The Spectra. of Hydrogen, Deuterium, Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen and Fluorine. By Charlotte E. Moore. (Circular of the National.
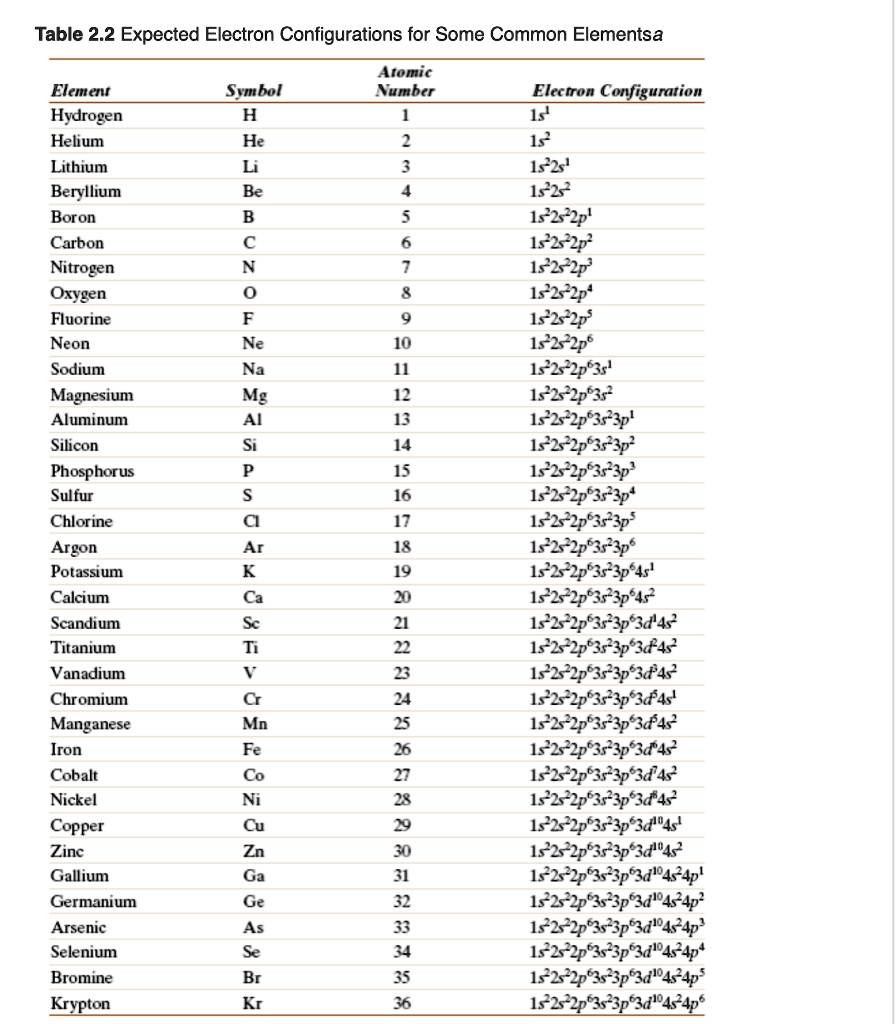
SOLVED Table 2.2 Expected Electron Configurations for Some Common Elements Atomic Number
Lithium (Li) is an alkali metal with atomic number 3, occurring naturally in two isotopes: 6 Li and 7 Li. The two make up all natural occurrence of lithium on Earth, although further isotopes have been synthesized. In ionic compounds, lithium loses an electron to become positively charged, forming the cation Li +.

The most abundant element present in the plants is
Atomic number color: red=gas. The noble gases (historically also the inert gases, sometimes referred to as aerogens [1]) are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these chemical elements are odorless, colorless.

7. Draw the electronic configuration of hydrogen, helium, lithium, beryllium, boron, carbon
5: The Electronic Structure of Atoms

7. Draw the electronic configuration of hydrogen, helium, lithium, beryllium, boron, carbon
The origin and evolution of lithium-beryllium-boron is a crossing point between different astrophysical fields:. This paper is a compliment to the previously published comparison of GCR models with AMS hydrogen, helium, and the boron-to-carbon ratio (Norbury et al., 2018). Detailed study of the astrophysical direct capture reaction There's Hydrogen and Helium Then Lithium, Beryllium Boron, Carbon everywhere Nitrogen all through the air With Oxygen so you can breathe And Fluorine for your pretty teeth Neon to light.
⚗️Write the electron configurations for the first 10 elements on the periodic table